Forte Van Der Waals
The equation consist of.
Forte van der waals. In molecular physics the van der waals force named after dutch scientist johannes diderik van der waals is a distance dependent interaction between atoms or molecules. En physique et en chimie une force de van der waals interaction de van der waals ou liaison de van der waals est un potentiel interatomique du à une intera. Intre moleculele ce alcatuiesc o substanta indiferent de starea ei de agregare se manifesta forte de interactiune aceste forte de interactiune confera fiecarei substante o energie de interactiune specifica sistemului molecular explicand astfel existenta starilor de agregare ale materiei. Like ionic or covalent bonds these attraction forces do not result from a chemical bond as they are comparatively weak and hence can be easily disturbed.
Referințe modificare modificare sursă despre forțele van der waals accesat pe 18 august 2015. Van der waals forces include attractive forces arising from interactions between the partial electric charges and repulsive forces arising from the pauli exclusion principle and the exclusion of electrons in overlapping orbitals. When the distance is less than 0 4 nm the net effect of the forces is repulsive as electron clouds repel each other. Their strength typically ranges from 0 4 kilojoules per mole kj mol to 4 kj mol and acts over distances of less than 0 6 nanometers nm.
Van der waals equation is required for special cases such as non ideal real gases which is used to calculate an actual value. The van der waals force was named after a dutch scientist johannes diderik van der waals 1837 1923. Guidebook on molecular modeling in drug design 1996. Tipuri de forte de interactiune moleculara.
They are comparatively weak and therefore more susceptible to disturbance. The forces are named for the dutch physicist johannes diderik van der waals who in 1873 first postulated these intermolecular forces in developing a theory to account for the properties of real gases. Definitia si rolul fortelor van der waals. This is considered as the first type of intermolecular forces between atom and molecules.
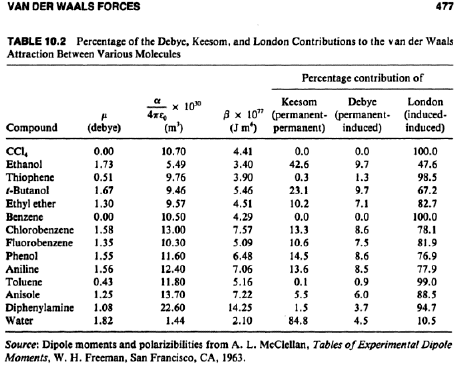
1 p n 2 a v 2 v n b n r t the v in the formula refers to the volume of gas in moles n. Van der waals forces are the weakest intermolecular forces.




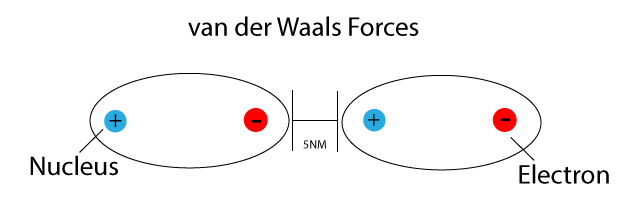


/91560215-56a133565f9b58b7d0bcfb65.jpg)